The Fight Against Lung Cancer
Developments in liquid biopsies provide significant implications in the advancement of care for patients with lung cancer. Lung cancer currently stands as the leading cause of cancer mortality with annual deaths exceeding those of breast, prostate, and colon cancers combined. Yet, most cases are not diagnosed until the disease progresses to later, more advanced stages when treatment options become limited.
Diagnosing Lung Cancer
Generally, diagnoses require the patient undergo a tissue biopsy, where a lung tissue sample is obtained from the patient for further analysis. Many factors must be considered for performing the procedure:
- Genetic marker variability
- Tumor accessibility
- Tissue supply
- Surgical risks
- Patient’s current health
- Cost
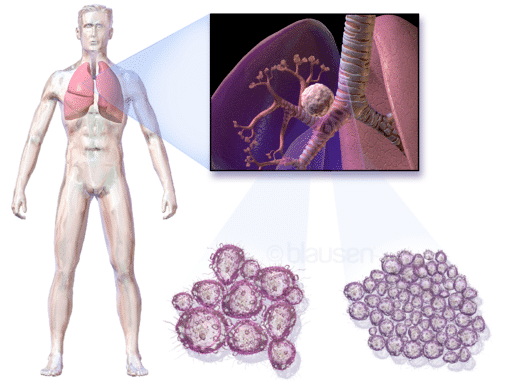
Diagnosing the disease at an early stage provides the most potential for successful treatment. Consequently, detecting circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) for these diagnoses are limited by the low concentrations found in blood at early stages. The issue becomes more complicated by another limitation of tissue biopsies: the temporary nature of the sample can warrant further procedures on the patient.
Witnessing Change
The development of liquid biopsies has fostered a means of obtaining the information needed to aid in a faster, simpler diagnosis and possibly faster prognosis.
In 2016, the Food and Drug Administration (FDA) approved the first liquid biopsy test. Originally intended for the blood plasma screening of patients with metastatic non-small cell lung cancer (NSCLC) with possible mutations in the epidermal growth factor receptor (EGFR) gene, the technique has since branched to many other lung cancer situations.
Today, liquid biopsies have further demonstrated increasingly therapeutic potential in detecting ctDNA and CTCs within body fluids. The procedure provides clinicians and patients with a minimally invasive screening which can provide early diagnosis results, identification of molecular markers, and insight into the tumor over time.
Behind the DNA
Unlike traditional biopsies, which require tissue samples, liquid biopsies detect ctDNA or CTCs within extracted body fluids such as blood and occasionally cerebrospinal fluid, saliva, or urine. The ctDNA is then amplified using the Polymerase Chain Reaction (PCR) procedure to detect the markers of interest using fluorescence or alternative means. Oncologists and researchers foresee several advantages with the rise in use of the technique:
Pros of Liquid Biopsy
- Minimal invasiveness
- Convenience
- Feasibility for serial procedures
- Monitoring changes in tumors throughout treatment
- Testing treatment effectiveness
- Assessing candidacy for clinical phase drugs

Challenges for the Future
As with any treatment, many factors must be considered for liquid biopsies and further clinical development is critical to improve efficacy. Studies have shown the ctDNA sensitivity for the tests may require sensitivity improvements to increase reliability for early-stage detection. For example, elevated antigen levels of certain markers may not always be indicative of a developing cancer. Moreover, cost and insurance coverage will require consideration to provide affordable testing potentially accessible in developing nations. Finally, a deeper understanding of molecular markers and variation in mutations within lung cancer types is essential to fully take advantage of the procedure.
Yet, in the context of a rapid complementary treatment with the potential to significantly impact high-risk patients during early stages of the disease, liquid biopsies present themselves a strong contender in the line of precision and genomic therapeutics against lung cancer.
Right: Lung Tissue
BioChain: Ease and Convenience
BioChain is proud to offer a range of specialized products designed to extract cfDNA in blood plasma or serum. The magnetic bead-based product can perform with less than 1 mL of a sample due maximizing sensitivity of cfDNA. In fact, BioChain’s kit recovers more cfDNA per mL of plasma when compared to competing products, possesses convenient automation compatibility, and is strictly tested for consistency in results.
The extraction kit yields results for Next Generation Sequencing, and the scalable system allows the sample to be conserved and used only as needed. The significantly cost-effective method can be utilized for further applications, from prenatal genetic testing to early cancer diagnoses. Compared to traditional biopsy methods, BioChain’s cfDNA extraction kits can provide a more efficient method of extraction from smaller fragmented chains.
The vamPure Blood Kit also provides a method of DNA extraction from whole blood samples. The advantage of this kit is that the buffer used is free from EDTA for specific experiments. EDTA can be added separately for long-term storage. This kit also supports automation.
As well as the kits, BioChain independently provides a PCR Purification Kit, which increases the quality of a DNA sequence by removing enzymes, salts, buffers, and short DNA fragments. This DNA amplification kit is compatible with automation methods, saving time and costs. Leading at the forefront of biotechnological advancement, BioChain continues to couple premier therapeutic products with competitive pricing to provide an exceptional clinical and research experience.
References
- Harris, Richard. When Genetic Tests Disagree About Best Option For Cancer Treatment. NPR Science Desk. Web. Mar. 2018.
- Gen News. Liquid Biopsy Predicts Colon Cancer Recurrence. Genetic Engineering and Biotechnology News. Web. Mar. 2018.
- Kuderer, N, et al. Comparison of 2 Commercially Available Next-Generation Sequencing Platforms in Oncology. JAMA Oncology. 2017;3(7):996–998. doi:10.1001/jamaoncol.2016.4983. Mar. 2018.
- Mone, Amy. Liquid Biopsy Results Differed Substantially Between Two Providers. John Hopkins Medicine. Web. Mar. 2018.
- Parnell, T. When Tests Collide: Perspectives on Liquid vs. Tissue Biopsies. MD News. Web. Mar. 2018.
- Shtivelman, E. Testing for Tumor Mutations: Liquid Biopsy Versus Traditional Biopsy. Cancer Commons. Web. Mar. 2018.
- Zhang, Sarah. One Patient, Two Cancer DNA Tests, Two Different Results. The Atlantic Monthly Group. 2016. Web. Mar. 2018.
Author
BioChain Institute Inc.

