Cancer Biomarkers
Biomarker - any biological signal that can be used to inform clinicians about a particular disease.
You’ve probably heard the term “biomarker” at some point while exploring current trends in cancer treatment. But what does the term really mean? The World Health Organization (WHO) has defined a biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. Essentially, a biomarker is any biological signal that can be detected and used as information for treatment purposes. This is particularly relevant for cancer research where the biology is often complex and unique for each patient and cancer type. Biomarker information obtained from diagnostics of patient biopsies can help distinguish a cancer’s unique signature profile. This information can be extremely useful in distinguishing a patient’s cancer risk, prognosis, malignancy and response to therapy (Ludwig and Weinstein 2005).
A Variety of Biomarkers from DNA to Protein
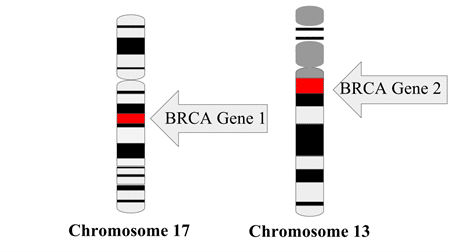
The first cancer biomarker was discovered by Dr. Joseph Gold in 1965. He identified a particular antigen, now known as carcinoembryonic antigen (CEA) that was highly elevated in the blood of patients with colon cancer. This biomarker is now used in hospitals for diagnosis and prognosis of colon cancer. Later in the 1990s, the Human Genome Project facilitated the discovery of genetic biomarkers, such as breast cancer associated genes BRCA1 and BRCA2, which provided insight into a patient’s predisposition to certain cancers. Since then, technological advancements have allowed a wider array of detectable biomarkers which now include RNA nucleotides (ie. microRNA or other non-coding RNA), gene and proteomic expression profiles, as well as metabolomic signatures.
Old & Emerging Technologies for Biomarker Detection
Detection of protein biomarkers typically rely on established immunoassays such as ELISA, protein microarrays and flow cytometry. These techniques measure the amount of biomarker present in a sample through binding of a labeled antigen. Though these methods are reliable and sensitive, they can be only used to detect established biomarkers. Recently, more advanced techniques have opened the door for comprehensive proteomic analysis such as mass spectrometry and two-dimensional electrophoresis (Lee et al. 2018). The ability to analyze signature proteomic profiles of various cancer subtypes could help identify of new and valuable biomarkers. Technological advances have also extended to cancer genetic profiling which historically relied on Sanger sequencing by capillary electrophoresis. Despite the reliability and high accuracy of Sanger sequencing, the technology was costly, labor-intensive and unsuitable for large-scale projects. These limitations have been addressed by next generation sequencing (NGS) technologies (right) which have allowed for more cost-effective, versatile and high-throughput analysis of both DNA and RNA transcriptomes (Kamps et al. 2017). Applications of NGS technology could help identify novel genetic biomarkers as well as provide more comprehensive genetic profiles of tumor subtypes.

Solid vs. Liquid Biopsies
Biomarkers are analyzed from either solid or liquid biopsies. Solid biopsies are taken directly from a tumor and analyzed for their genetic makeup and the presence of certain biomarkers. Unfortunately, there are several drawbacks to solid biopsies as the extraction can be risky, painful and sometimes impossible depending on tumor accessibility. Meanwhile, liquid biopsies can provide valuable information on disease progression while being non-invasive, painless and cost efficient. Liquid biopsies are taken primarily from whole blood but can take the form of any non-solid biological tissue including serum, plasma, stool or urine. A large proportion of liquid biopsies are used to analyze circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) that have shed from the primary tumor and are detected in the blood stream and other peripheral fluids. Analysis of CTCs and ctDNA relies on proper isolation of cell-free DNA (cfDNA) or DNA that is non-encapsulated in the bloodstream (Marrugo-Ramírez, Mir, and Samitier 2018).
Read: A Tale of Two Biopsies: Liquid Biopsy vs Tissue Biopsy
BioChain offers Diverse, Convenient & Published TMAs for Biomarker Research
Identifying novel biomarkers requires screening in a wide variety of tumor types which can be time consuming, costly and technically difficult. BioChain offers tissue samples of several tumor types in a microarray (TMA) format for rapid and cost-efficient biomarker screening. For instance, BioChain’s breast cancer tissue array (right) provides samples from 70 duplicated cases covering all common types of breast cancer. The samples are provided with comprehensive TNM staging and biomarker data that includes HER2, estrogen receptor (ER) and progesterone receptor (PR) status. No matter what your research needs are, BioChain can assist with their proven, versatile and cutting-edge research tools.
Rapid and convenient cfDNA purification by BioChain
BioChain proudly offers a variety of cfPure® liquid biopsy kits (right) for purification of cell free DNA (cfDNA) for your research needs. The kits are automation friendly and magnetic bead-based which allows for rapid and efficient isolation of low molecular weight cfDNA for various downstream analysis such as qPCR and NGS.

References
- Kamps, Rick et al. 2017. “Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification.” International Journal of Molecular Sciences.
- Lee, Chan-Hyeong, Eun-Ju Im, Pyong-Gon Moon, and Moon-Chang Baek. 2018. “Discovery of a Diagnostic Biomarker for Colon Cancer through Proteomic Profiling of Small Extracellular Vesicles.” BMC Cancer.
- Ludwig, Joseph A., and John N. Weinstein. 2005. “Biomarkers in Cancer Staging, Prognosis and Treatment Selection.” Nature Reviews Cancer.
- Marrugo-Ramírez, José, Mònica Mir, and Josep Samitier. 2018. “Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy.” International Journal of Molecular Sciences.
Author
BioChain Institute Inc.

