
The Power of Liquid Biopsy
Cancer has long remained a major cause of death in the United States. Billions of dollars are being spent every year in trying to find a cure which has eluded researchers for decades. The field of liquid biopsy may be the missing link for effective diagnosis and treatment methods, going a long way to finding the ultimate “cure” for cancer and other diseases.
Using the Diversity of Fluids for Biopsy
A liquid biopsy starts with examining a sample of fluid from the body which may include blood, urine, saliva, and cerebrospinal fluid.Blood contains a mixture of various substances including red blood cells, white blood cells, platelets, exosomes, RNA, DNA, and proteins. The plasma is obtained by centrifuging blood which can further be simplified into serum. The serum refers to the part of the plasma that lacks clotting agents because of its coagulated nature, and thus simplifies the liquid to focus on what is needed for a biopsy. While the majority of liquid biopsies utilize blood, urine can prove extremely useful, particularly for cancers relating to the endocrine system.
Exosomes are vesicles from blood cells that may be used for diagnostic purposes due to the presence of DNA, RNA, and proteins from their original cell.Such nanoparticles have been found to spread cancer throughout the body by travelling in the blood and fusing with healthy cells at distant sites. Therefore, by targeting exosomes directly, further metastases may be prevented. According to San Lucas et al., exosomes contain high quality DNA that shows 90-95% of the same genetic markers as the origin tumor, and thus are useful in the diagnosis and tracking of cancer. Exosomes, along with blood, can also be found in urine and used in the diagnosis and tracking of various cancers including prostate, kidney, and colorectal cancer (Nilsson et al.).
As a vital component of liquid biopsies, messenger RNA (mRNA) found in urine may be used in the diagnosis of kidney, bladder, and prostate cancers (Di Meo et al.). RNA has long been hypothesized to have evolved before DNA, but it is primarily DNA that tends to be used in liquid biopsies. While DNA is often found in the cell nucleus and other organelles such as mitochondria, it is not associated with any cells in the blood plasma.

A tumor is a growth of cells that may or may not be cancerous. It is theorized that when cell death occurs, some of the cellular DNA begins circulating in the blood and other fluids as cell-free DNA (cfDNA). Some of the various forms of cfDNA in the blood include cell-free fetal DNA (cffDNA), donor derived cell free DNA (ddcfDNA), and circulating tumor DNA (ctDNA). This ctDNA is often the most important element in a liquid biopsy for cancer due to the ability to track the DNA to the organ of origin and reliability provided for locating solid tumors (Karachaliou et al.). Further research by Bettegowda et al. has demonstrated that both metastatic and localized cancers are capable of detection through ctDNA fragments in the blood. All of these various types of DNA work with liquid biopsies due to a process called Next Generation Sequencing, which will be examined later in detail.
Liquid biopsies are useful in diagnoses, treatments, transplants, and prenatal health. The next section will provide insight into some of these applications.
The Versatility of a Liquid Biopsy
Despite the liquid biopsy initially being considered revolutionary for its early and simple diagnosis of cancer, its versatility extends to many other applications. As a result, cancer is not the only priority for the oncological test.
For example, cffDNA, or DNA that originates from a fetus, can be found in a mother’s body and examined in liquid biopsies (Chitty & Lo). Referred to as Non-Invasive Prenatal Testing (NIPT), the analysis can be used to screen for chromosomal abnormalities such as Down’s Syndrome, Edward’s Syndrome, and Patau Syndrome. If abnormalities are found, a more invasive method (e.g. amniocentesis or chorionic villus sampling) will likely be recommended to form a diagnosis.
The scope of liquid biopsies further extends to organ transplants. As Hofste et al. note, transplanted organs release donor-derived cell free DNA (ddcfDNA) in the body. The amount of the ddcfDNA can be compared to the body’s original cfDNA in a liquid biopsy using blood. The results can then help determine whether the body is accepting or rejecting a graft in the early stages of a transplant and even help determine immunosuppression levels that will ensure transplant success.

Liquid Biopsies in Oncology
Oncology has long been the primary field of liquid biopsies with regards to both diagnosis and treatment.
Precision, or personalized, medicine is a subfield in oncology care that focuses on the patient’s genetic profile to target their cancer. Molecular profiling assesses cfDNA to find markers deemed significant which are then used in treatment (Di Meo et al.). Unfortunately, not all cancer is the same, and thus, blanket treatments may cause more harm than good. Utilizing a liquid biopsy can help identify if a treatment is effective and whether or not it should be continued. As mentioned earlier, exosomes may be one of those targets for treatment that may prevent the advance of cancer.
Tumors can be unaffected by treatment using DNA associated with resistance in the past. In fact, Helwick reports in one study that resistance was successfully identified in 27% of patients using a liquid biopsy. Acquired resistance is also a major obstacle to eradicating cancer with the use of drugs and treatments, such as radiation, that have severe side effects. Using specific genetic mutations from ctDNA, a process called DNA sequencing can decrease associated dangers of such treatment.
T-cell transfer immunotherapy is a treatment in which specific T-cells are collected from a patient, cultured in the lab, then returned back into the patient’s body in increased numbers to fight the cancer. Xi et al. demonstrated in their research regarding the procedure that ctDNA could be used to determine whether a treatment was effective within a mere two weeks. The test’s rapid determination of treatment effectiveness will aid patients from further continuing potentially ineffective and painful treatments.

Applications of a Liquid Biopsy in Oncology (Bettegowda et al.)
Workflow for Breast Cancer
The application of liquid biopsies employs Next Generation Sequencing (NGS), a specific type of Whole Genome Sequencing (WGS) which arranges and maps the DNA of an organism’s entire genome within a day (Behjati & Tarpey). Fragments of DNA are processed multiple times to provide an extremely detailed and accurate overview of exomes, which comprise a fraction of the genome. Using NGS, hundreds of different breast cancer tumors have been sequenced. The results revealed previously unknown mutations in DNA that may be associated with breast cancer in potential patients (Desmedt et al.). Such mutations are divided into driver mutations (those that contribute to the development of a tumor) and passenger mutations (those that can change through the course of cancer treatment into resistant clones of a tumor).
Extensive research using 20,592 women with breast cancer has helped map out an entire panel of genes to be tracked through liquid biopsies, as reported by O’Leary et al. These genes include mutations leading to breast cancer, common gynecologic cancers, and other tumors. Abnormal methylation, an epigenetic aberration that may indicate a marker for breast cancer, can further be detected by liquid biopsies in cfDNA during the diagnosis process. The potential of liquid biopsies can be illustrated in this regard by Petr et al. through a study which identified 3,506 methylated regions within the examined breast cancer.
After a diagnosis is made, treatments can be decided and initiated using the aforementioned techniques of precision medicine. During the process, liquid biopsies can serve to determine the efficacy and efficiency of the treatment. Even after a patient has reached remission, liquid biopsies prove absolutely vital as a screening tool to track and manage the return of the breast cancer.
A Long Needed Relief for At-Risk Demographics
A focus on the future of liquid biopsies, due to their large candidate eligibility, continues to make them an increasingly available and accessible screening tool for the general population. While the general population certainly stands to benefit from liquid biopsies, one demographic in particular stands to gain the most: cancer patients.
As Miller et al. reported in 2016, there were approximately 15.5 million cancer survivors in the United States alone, a number that is rapidly increasing as technology and treatment methods improve. While the demographic is at an increased risk for relapse, liquid biopsies serve to provide patients and providers with a cheap and non-invasive alternative to regularly test for the return of cancer.
Further populations that are vulnerable also need a method that will not compromise their immune system or extend recovery time. For example, Ilie et al. observed that liquid biopsies detected lung cancer early using ctDNA in those with chronic obstructive pulmonary disease (COPD), a condition which prevents a traditional diagnostic method such as tissue biopsy from being used. Similarly, Mischak observed that in patients with Chronic Kidney Disease (CKD), liquid biopsies can provide a less intrusive alternative to the usage of kidney tissue to track certain proteins.
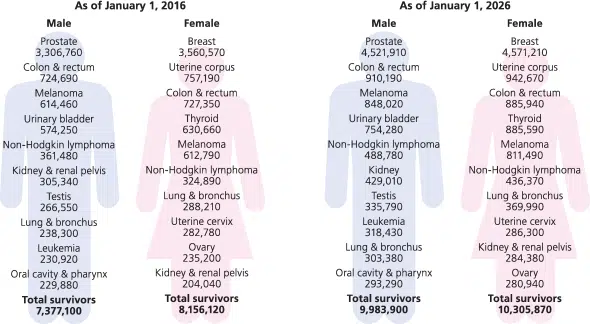
Estimated Survivors of Cancer in 2016 and Projected Survivors in 2026 (Miller et al.)
It is important to note that at their current stage, liquid biopsies should be used in conjunction with traditional methods as necessary rather than solely. The next section will discuss how each form of biopsy has its own advantages and disadvantages.
A Long Needed Relief for At-Risk Demographics
Traditional biopsy methods can be invasive or dangerous for patients, often qualifying as surgery, and can include tissue biopsies (tissue, marrow), imaging scans (x-ray, MRI, ultrasound), and lab tests (blood, urine).
Disadvantages of Traditional Biopsy Methods
- Medical imaging procedures often expose the patient to dangerous elements (e.g. radioactive materials). While generally not of concern in imaging situations, repeated exposure of such radiation when constant information of the cancer is necessary may result in a weakening body, even for patients in relapse.
- Tissue biopsies involve extracting relevant samples of matter from the body. These tissues may come directly from a tumor, such as in breast or skin cancer, or may be samples collected from bone marrow. While a specialized needle is often used to complete extraction, endoscopic methods may be used to extract tissue from unreachable areas, such as the lungs or colon, albeit in a manner perceivably more painful to the patient.
- Should the tumor be located in an unreachable location due to another disease, as noted with COPD and CKD, tissue biopsy methods cannot be used to diagnose and track the tumor. Repeated use of traditional biopsy or alternative treatments may also cause long-term damage to patients in a weak state of health. This point may be especially salient when discussing cancers in children. Constant taxing methods of tracking tumors may further increase risks for diseases in the future. For example, research by Armstrong et al. notes that 50% of childhood cancer survivors develop long-term chronic illness by the age of fifty.
- Traditional biopsies further tend to be limited in use. As presented by Baldwin of the American Society for Clinical Oncology, liquid biopsies can find ctDNA, even if levels present are less than 0.4% of circulating DNA in the blood. Finally, the results of a liquid biopsy can be provided in as little as two weeks, compared to a month for those of tissue biopsies.
Disadvantages of Liquid Biopsy Methods
- One disadvantage of the liquid biopsy is its tendency to give greater false positives than traditional biopsies do. A false positive occurs when the sample indicates cancer, even if no danger is actually present – either because the tumor has already been caught and eradicated by the immune system or because it continues to grow so slowly that it will not harm the patient in his or her lifetime. False positive results can lead to overtreatment, which may do more damage than good in the long run.
- Another disadvantage is how liquid biopsies can detect ctDNA, but not necessarily the point of its origin. Therefore, even if a tumor is present somewhere in the body, it may be difficult to determine its location without extensive follow-up testing. As Collins notes, however, machine learning can greatly narrow down the organ of origin as demonstrated in the majority of cases.
- While liquid biopsies are strong therapeutic alternatives, there remains some time until their full potential is achieved.
The Future of Liquid Biopsies
One of the highest priorities for liquid biopsies is increased commercialization to make the tool as cost-effective and widely available as possible. Volik et al. report that great progress has been made in commercializing liquid biopsies for cancer diagnoses.
In 2016, the FDA approved the first liquid biopsy test for lung cancer. Since then, the field has exploded with innovation: applications from fetal testing and organ transplantation to genome mapping show that liquid biopsies have a scope far beyond oncology. Even within the field of cancer research, multiple ongoing studies continue to demonstrate the superiority of liquid biopsies over traditional methods.
For example, work is being done on a test that spots both DNA and protein biomarkers for pancreatic cancer. While the test is not yet ready for commercialization, it has been claimed that mutations in DNA shed from cancer cells and found in the blood is “exquisitely specific” for cancer. “If cancer-linked DNA can be found in the blood of an individual, it is very likely that person has cancer,” says Vogelstein, co-director at the Johns Hopkins Kimmel Cancer Center. Studies by the clinical research center and others have shown more than 85% of patients with advanced cancer have identifiable DNA in their blood. Today, the possibility of identifying such small pieces of DNA in the blood without prior knowledge of the genetic status of the cancers demonstrates how quickly the field is advancing.
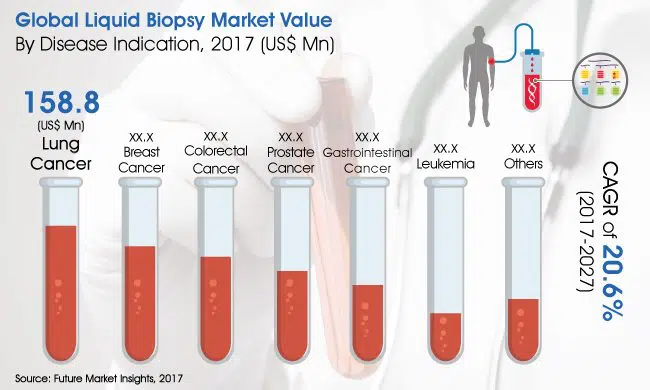
Key Trends in the Liquid Biopsy Market Value (Future Market Insights)
A current ongoing research study done by the Stanford Cancer Institute and the National Cancer Institute is set to determine the effectiveness of genomic profiling in precision treatments. Further research studies continue to focus on being able to identify markers for diseases identified by a biopsy.
BioChain: A Leader in Liquid Biopsy Sample Preparation
BioChain is proud to offer a range of specialized products designed to extract cfDNA in blood plasma or serum. The magnetic bead-based product can perform with less than 1 mL of a sample despite being able to maximize sensitivity of cfDNA. In fact, BioChain’s kit recovers more cfDNA per mL of plasma when compared to competing products, possesses convenient automation compatibility, and is strictly tested for consistency in results.
Uses of the extraction kit range from NGS to PCR for DNA amplification while the scalable system allows the sample to be conserved and used only as needed. The significantly cost-effective method can be utilized for further applications, from prenatal genetic testing to early cancer diagnoses. Compared to traditional biopsy methods, BioChain’s cfDNA extraction kits can provide a more efficient method of extraction from smaller fragmented chains. Leading at the forefront of biotechnological advancement, BioChain continues to couple premier therapeutic products with competitive pricing to provide an exceptional clinical and research experience.

References
- Armstrong, Gregory T. et al. “Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer.” Journal of Clinical Oncology 31.29 (2013): 3673–3680. PMC. Web. 28 Feb. 2018.
- Baldwin, K. “Liquid Biopsy May Help Guide Treatment Decisions for Advanced Solid Tumors.” American Society of Clinical Oncology. (2016). Web. 28 Feb. 2018.
- Behjati, Sam, and Patrick S Tarpey. “What Is next Generation Sequencing?” Archives of Disease in Childhood. Education and Practice Edition 98.6 (2013): 236–238. PMC. Web. 28 Feb. 2018.
- Bettegowda, Chetan et al. “Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies.” Science translational medicine 6.224 (2014): 224ra24. PMC. Web. 28 Feb. 2018.
- Chitty, Lyn S., and Y. M. Dennis Lo. “Noninvasive Prenatal Screening for Genetic Diseases Using Massively Parallel Sequencing of Maternal Plasma DNA.” Cold Spring Harbor Perspectives in Medicine 5.9 (2015): a023085. PMC. Web. 28 Feb. 2018.
- Collins, F. “New ‘Liquid Biopsy’ Shows Early Promise in Detecting Cancer.” National Institutes of Health. Web. 28 Feb. 2018.
- Desmedt, Christine et al. “Next Generation Sequencing in Breast Cancer: First Take Home Messages.” Current opinion in oncology 24.6 (2012): 597–604. PMC. Web. 28 Feb. 2018.
- Di Meo, Ashley et al. “Liquid Biopsy: A Step Forward towards Precision Medicine in Urologic Malignancies.” Molecular Cancer 16 (2017): 80. PMC. Web. 28 Feb. 2018.
- FDA News Release. “FDA approves first blood test to detect gene mutation associated with non-small cell lung cancer.” U.S. Food and Drug Administration. 2016. Web. 28 Feb. 2018.
- Future Market Insights. “Liquid Biopsy Market: Rising Cases of Lung Cancer Fuelling Adoption of Liquid Biopsy Across the World: Global Industry Analysis (2012 - 2016) and Opportunity Assessment (2017 - 2027).” Future Market Insights. Web. 28 Feb. 2018.
- Helwick, Caroline. “‘Liquid Biopsy’ Stacks Up Well to Tissue Biopsy in Detecting Tumor-Specific Mutations.” The ASCO Post. Harborside Press. Web. 28 Feb. 2018.
- Hofste, L et al. “Liquid Biopsies: Non-invasive Rejection Detection After Heart Transplantation.” The Journal of Heart and Lung Transplantation 36. 4 (2017). Web. 28 Feb. 2018.
- Ilie, Marius et al. “‘Sentinel’ Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease.” Ed. Vladimir V. Kalinichenko. PLoS ONE 9.10 (2014): e111597. PMC. Web. 28 Feb. 2018.
- John Hopkins Medicine. “Combined DNA and Protein ‘Liquid Biopsy’ for Early Pancreatic Cancer Better Than Either Alone.” The John Hopkins University. (2017). Web. 28 Feb. 2018.
- Karachaliou, Niki et al. “Real-Time Liquid Biopsies Become a Reality in Cancer Treatment.” Annals of Translational Medicine 3.3 (2015): 36. PMC. Web. 28 Feb. 2018.
- Miller, K et al. Cancer Treatment and Survivorship Statistics, 2016. CA: A Cancer Journal for Clinicians 66: 271–289. doi:10.3322/caac.21349. Web. 28 Feb. 2018.
- Mischak, H. “Pro: urine proteomics as a liquid kidney biopsy: no more kidney punctures!” Nephrol Dial Transplant 30 (4): 532-7. doi: 10.1093/ndt/gfv046. Web. 28 Feb. 2018.
- National Cancer Institute. “Genomic Profiling in Recommending Treatment for Patients with Metastatic Solid Tumors.” National Cancer Institute at the National Institutes of Health. Web. 28 Feb. 2018.
- Nilsson, J et al. “Prostate Cancer-Derived Urine Exosomes: A Novel Approach to Biomarkers for Prostate Cancer.” British Journal of Cancer 100.10 (2009): 1603–1607. PMC. Web. 28 Feb. 2018.
- O’Leary, Erin et al. “Expanded Gene Panel Use for Women With Breast Cancer: Identification and Intervention Beyond Breast Cancer Risk.” Annals of Surgical Oncology
Author
BioChain Institute Inc.

