Introduction: Comparison of Three Circulating Cell-Free DNA Extraction Methods
Since the late 1990s, circulating cell-free DNA (ccfDNA) has become an increasingly important and well-studied biomarker for cancer diagnostics (1), transplantation rejection (2), prenatal diagnosis (3) and other medically important biological processes. While diagnostic methods that employ this sample type were originally predominantly PCR-based, next generation sequencing (NGS) has become the diagnostic method of choice for analysis of ccfDNA is rapidly gaining acceptance as a powerful alternative (4). Therefore, purification protocols must produce ccfDNA of a concentration and purity that is amenable to use in NGS-based diagnostics. Additionally, any practical ccfDNA extraction needs to be applicable to a range of sample volumes - typically 0.5-5mLs, but potentially up to 10mLs of serum and/or plasma. Lastly, since diagnostic labs may need to process a range of samples, from roughly 10 to over 1000s per week, an ideal method is one that can be easily automated as throughput increases.
Historically, the QIAGEN QIAamp ccfDNA extraction kit is the most referenced commercial kit for this purpose. The yields and quality of ccfDNA extracted by this kit have been considered the standard by which any other kits have been judged. The drawback to this kit is that it uses a low volume silica-based purification column, combined with a larger detachable reservoir and a vacuum manifold. The combination of column and reservoir work well and are relatively easy to use for manual, low-throughput purposes. This methodology requires complex actions that are not amenable to automation. Additionally, since one column is used for each ccfDNA extraction, costs per extraction do not scale with sample volume. Extraction of 1mL of serum or plasma with incur the same materials and labor costs as the extraction of 5mLs or more. A second method is needed that allows for automation to reduce both labor costs and the potential for errors while extracting ccfDNA from large numbers of samples. Ideally this method would be scalable, so that material costs can be reduced if the input sample volume can be decreased. To satisfy the need for scalability and automatability, various suppliers have developed methods that use silica-coated magnetic beads instead of columns. Magnetic fields can then be used to move the beads from sample, to wash buffer, to elution buffer in automated systems without significant human intervention. Magnetic beads also provide for scalable purification systems. Reducing sample volume reduces the number of beads required for a purification.
In this study, we extracted ccfDNA from 10mLs of plasma using the column-based QIAamp ccfDNA purification kit, and two different commercially available ccfDNA extraction kits that employ magnetic beads. The ccfDNA purified by all three methods were evaluated for yield, fragment size, and performance based on low coverage whole genome NGS analysis.
Materials and Methods
Samples:
Sufficient plasma was collected from four healthy donors to perform in triplicate ccfDNA purifications, using 10mLs of plasma per extraction using three different commercially available ccfDNA extraction kits (total 90mLs).
ccfDNA Purification:
Circulating cell free DNA was extracted by the column-based QIAGEN QIAamp Circulating Cell Free DNA purification kit (QIAGEN Inc, Hilden, Germany), the magnetic bead-based ThermoFisher MagMax cell-free DNA extraction kit (ThermoFisher Scientific, Waltham, MA) and the magnetic bead-based BioChain cfPure Cell Free DNA Purification kit (BioChain Institute, Inc., Newark, CA). In all cases, 10mL plasma samples were processed according to Manufacturers’ protocols.
Library Construction:
Libraries made by KAPA HyperPrep next generation sequencing library preparation kit (Kapa Biosystems, Capetown, SA), according to manufacturer’s directions, using Stubby-Y Adapters (IDT, IA) with 9 cycles of amplification using dual-unique indexing primers (IDT, IA). In all cases, 20ng of input ccfDNA was used.
Sequencing:
Whole-genome NGS libraries are quantified by Qubit broad range kit, and by qPCR using the KAPA Library Quantification Kit for Illumina® platforms (Kapa Biosystems). Fragment sizes were confirmed by 2100 Agilent BioAnalyser (Agilent Technologies, Santa Clara, CA). Following quantification, each library was subjected to 2x75 paired end sequencing, targeting 25M reads each on the NextSeq 550 mid-output sequencing system (Illumina Inc, San Diego, CA) according to manufacturer’s recommendations.
Sequence Analysis:
The sequencing libraries were analyzed for number of reads, insert size, mapping rate, distribution of reads per chromosome, whole genome coverage, and GC bias.
Results
Library Construction:
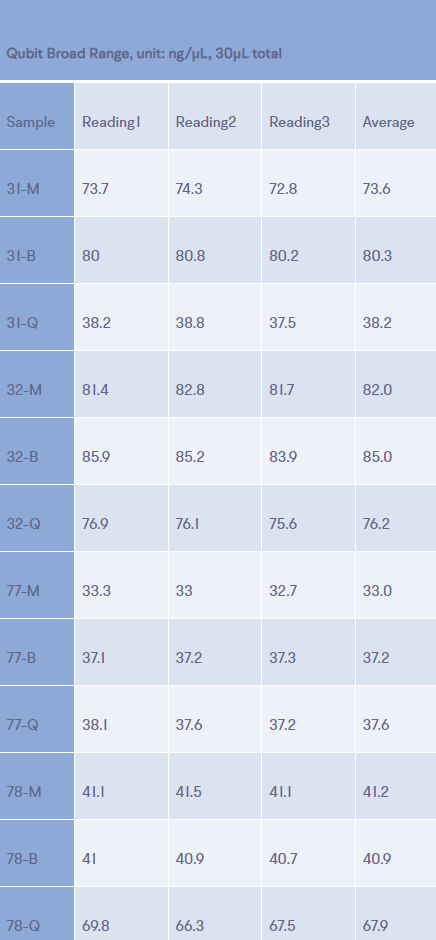
20ng of ccfDNA extracted from samples provided by four different donors, using the QIAGEN QIAamp Circulating Cell-Free DNA isolation kit (Q), the ThermoFisher MagMax Cell-Free DNA extraction kit (M), and the BioChain cfPure Cell-Free DNA isolation kit (B) were used to create next generation sequencing libraries using the Kapa Biosystems HyperPrep kit. Both Qubit fluorimetric and qPCR quantitation were used to assess library yields (Table 1). In all cases both quantitation methods yielded similar results for all twelve libraries. Additionally, with the exception of two of the ccfDNA samples extracted by the QIAamp kit, ccDNA extracted from the four different plasma samples yielded similar amounts of amplified library DNA. For two of the plasma samples, ccfDNA produced by the QIAamp kit, library yields were either significantly lower or significantly higher than those produced from ccfDNA produced by the other two methods.
Table 1a qPCR
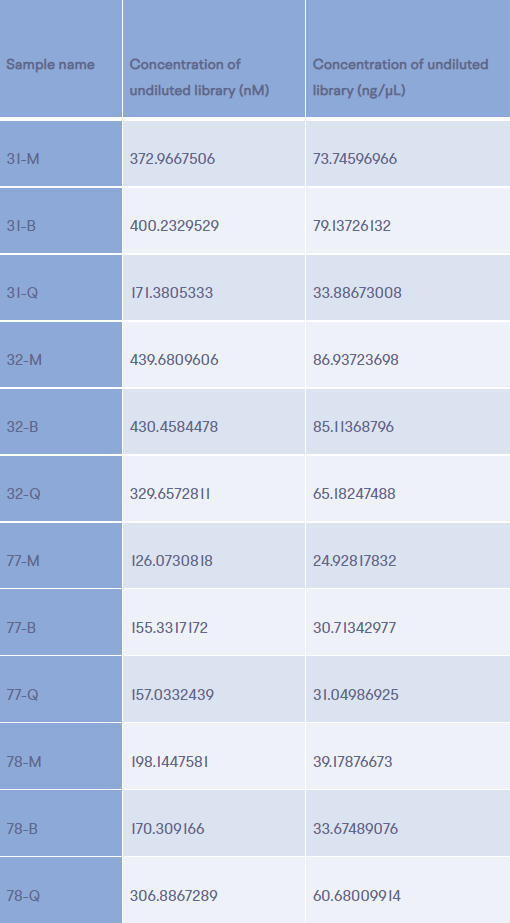
Table 1b Qubit
Sequence analysis:
In all cases, each library yielded more than 25 million reads when subjected to paired end sequencing (Table 2). Furthermore, all libraries in this study yielded inserts with an average length of 168-169 base pairs (Figure 1). All libraries yielded an average of approximately 97% mapped reads (Figure 2). The percentage of reads mapping to chromosomes 1-22, X, and Y were compared by averaging the results from sequencing ccfDNA from each of the four plasma samples extracted by each of the three extraction methods (Figure 3). An equivalent percentage of reads from ccfDNA isolated by each of the three ccfDNA extraction kits mapped to each chromosome. In many cases, analysis of ccfDNA will be done at low coverage to minimize the amount of input sample required. Accordingly, each library in this study was created from 20ng of input ccfDNA. In all cases, the mean coverage achieved was between 0.549X and 0.550X (Figure 4). Lastly, GC coverage was compared across regions that ranged from 0-19% GC to regions that ranged from 80%-100% GC. In all cases, there is no significant difference in GC coverage between each of the three extraction methods (Figure 5).
Figure 1
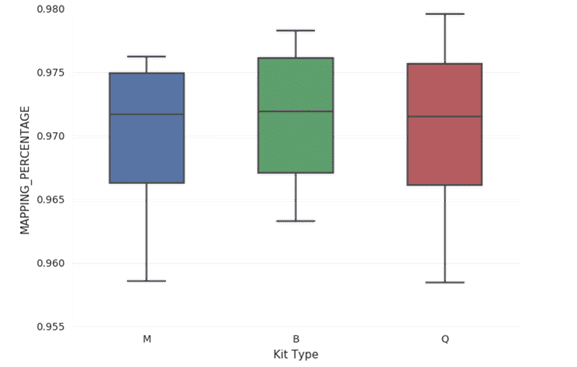
Figure 2
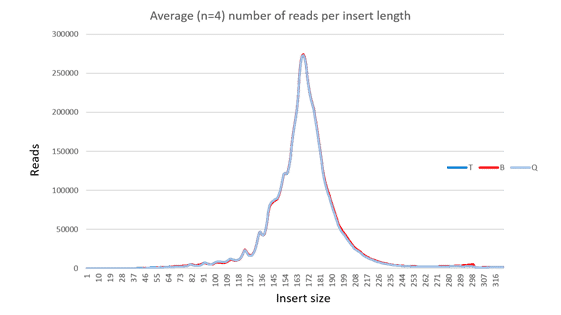
Figure 3
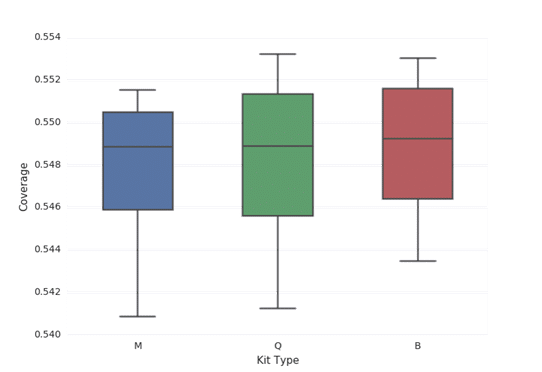
Table 1

Figure 4

Figure 5

Discussion
When column-based and magnetic bead-based circulating cell-free DNA (ccfDNA) extraction methods are used to extract ccfDNA from plasma, the purified nucleic acids are equally well-suited to whole genome sequencing. Bead-based methods provide additional advantages in terms of scalability, and are designed to be amenable to automation.
BioChain: Ease and Convenience
BioChain is proud to offer a range of specialized products designed to extract cfDNA in blood plasma or serum. The magnetic bead-based product can perform with less than 1 mL of a sample due maximizing sensitivity of cfDNA. In fact, BioChain’s kit recovers more cfDNA per mL of plasma when compared to competing products, possesses convenient automation compatibility, and is strictly tested for consistency in results.
The extraction kit yields results for Next Generation Sequencing, and the scalable system allows the sample to be conserved and used only as needed. The significantly cost-effective method can be utilized for further applications, from prenatal genetic testing to early cancer diagnoses. Compared to traditional biopsy methods, BioChain’s cfDNA extraction kits can provide a more efficient method of extraction from smaller fragmented chains.
References
- Schwarzenbach, H., Hoon, D., and Pantel, K., Cell-free nucleic acids as biomarkers in cancer patients. Nature Reviews (Cancer) (2011) volume 11, pages 426–437
- Burnham, P., Khush, K., De Vlaminck I., Myriad Applications of Circulating Cell-Free DNA in Precision Organ Transplant Monitoring. Ann Am Thorac Soc. (2017) Volume 14, pages S237-S241
- Taneja, P. et al. Fetal aneuploidy screening with cell-free DNA in late gestation. The Journal of Maternal-Fetal & Neonatal Medicine. (2017) Volume 30, Pages 338-342
- Cree, I., A. et al. The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: a systematic mapping review. BMC Cancer (2017) 17:697
Author
Geoffrey Routh, Ph.D.

