
An Introduction to Protein Extraction Methods
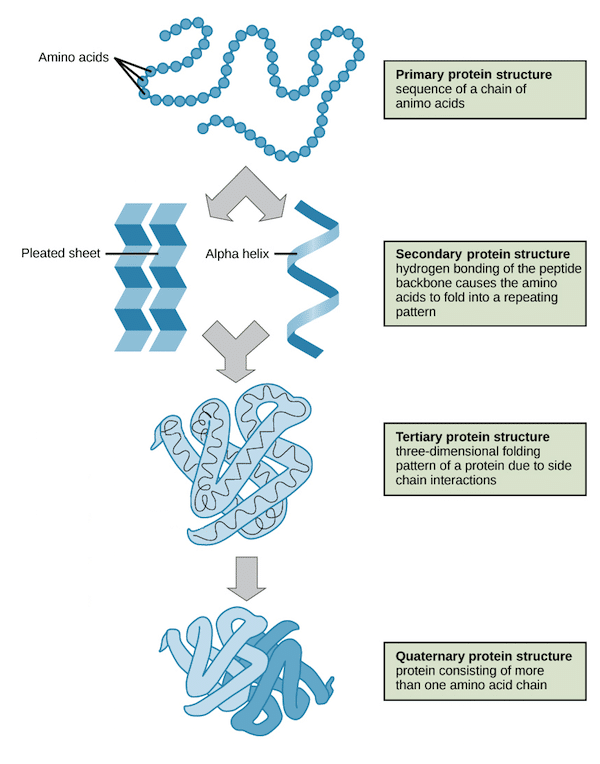
A protein is a macromolecule that consists of a chain of amino acid residues that complete vital functions in the body. Ranging from DNA replication to catalyzing chemical reactions to providing structural support, proteins complete infinite tasks in the body essential for survival of the organism. Proteins are often classified by their structure, which takes into account how many chains of amino acids form its physical shape and pattern. This is depicted in Figure 1. Every aspect of the body starting from individual cells, building up to tissues, and entire organs require a precise balance of proteins for optimal functioning. As a result, proteins are incredibly important to study in biotechnology. Figure 1: Four Orders of Protein Structures (Khan Academy)
The studying of proteins occurs in three major ways:
In Vivo: This method studies proteins within the whole of an organism, particularly to track how they interact with other parts of the body, and protein-protein interactions. In vivo studies are a necessary aspect of any clinical trial.
In Vitro: Proteins are purified and studied in controlled environments, such as in a lab or clinic. This helps isolate any confounding effects that can occur during the study. In vitro studies are vital to complete before using live models due to potential risks, particularly in drug testing.
In Silico: A more recent method, this studies proteins using computer simulations. Utilizing computer models decreases time, resources, and labor needed to complete certain projects. Cell analysis, including genome sequencing and protein folding, benefits greatly from in silico models. A sample of an in silico model is depicted in Figure 2.
There are certain proteins that are used more frequently in research due to how important they are in the body. They can be grouped into specific categories. Extracellular matrix proteins provide structural support to cells, such as elastin and collagen. Globular protein refers to a folding pattern and includes several varieties of proteins with various functions. Below are some of the more common types of globular proteins.
Methods of studying proteins almost always involve needing to purify the protein from other cellular components, then isolating it. This process has historically been a time-consuming and laborious one. The two major reasons proteins are purified are either for preparative use (producing large quantities of the same protein for use, such as insulin or lactase) or analytical use (extracting a small amount of protein to use in structural or functional research).
Figure 2: In Silico Methods may use Computational Modeling (Profacgen)

Uses of Isolated Proteins
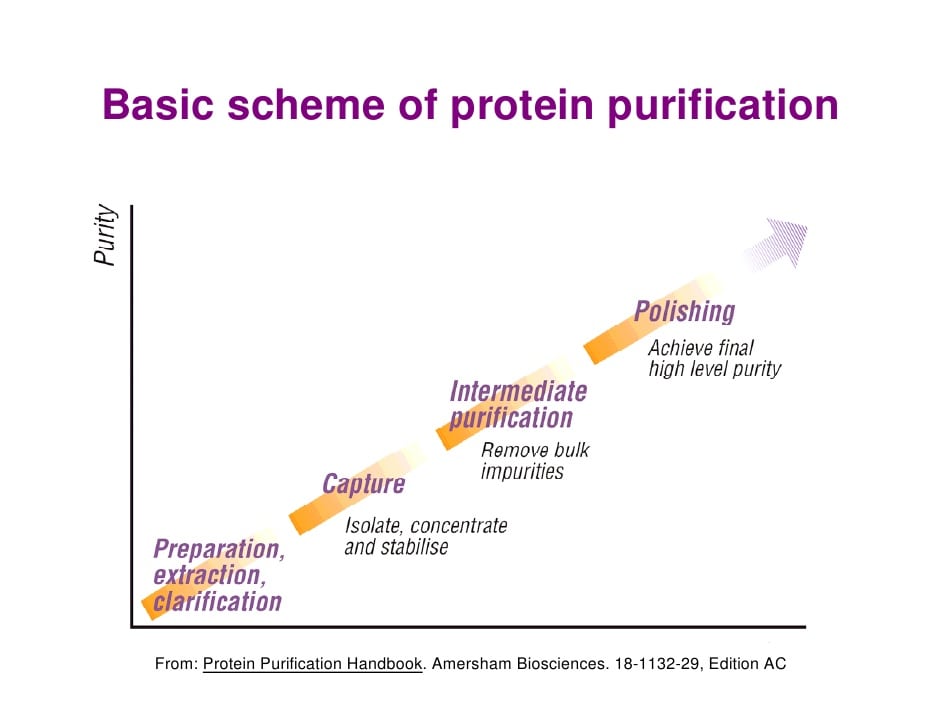
As one of the most widely studied molecules in biological research, protein extraction has vast applications. From commercial products to research, the first step tends to consist of completely purifying protein first. The process of protein purification requires many steps before the protein is usable, as depicted in Figure 3.
The detection of protein presence is a challenging area due to how small their size is. Total protein detection methods include absorbance, using amido black or colloidal gold, detecting nitrogen, and assays such as the Bradford Protein. Specific methods detect amounts present of a single protein. Chief among these are spectrometry and antibody dependent methods.
In clinical uses, the isolation of proteins can be used in diagnosing diseases. For example, the presence of insulin in the urine can be an indicator for diabetes. Furthermore, purified proteins are often vital in disease treatment or for cosmetic procedures, such as using collagen for skincare.
In research, there are several downstream applications to protein purification that require high quality polished extractions, several of which are detection methods.
In research, there are several downstream applications to protein purification that require high quality polished extractions, several of which are detection methods.
Immunoprecipitation (IP): This process creates an antigen from immobilized protein antibodies and solidifies it from a solution using a solid support. The technique initially developed using agarose resin, but modern purification procedures use magnetic beads due to increased speed, convenience, and automation. IP is frequently the first step before assay techniques or blotting.
Proteomics: The proteome of an organism describes the total set of proteins it can create or use. Proteomics studies in humans focus on identifying all of the proteins present in the human body, as well as their quantities, function, and structure. This helps find proteins of interest faster.
Enzyme Assay: Most often performed to either identify the presence of a specific enzyme or to determine the amount of total/specific enzymes present, assays are vital in measuring enzymatic activity. Experiments can be continuous or discontinuous, and often have to control precise confounding factors such as pH or temperature, as shown in Figure 4.
Assays are categorized specifically:
Relaxation Experiments: These experiments disturb the equilibrium of an enzyme solution to analyze the reactions occurring as the solution returns to equilibrium state.
Transient Kinetics Experiments: This difficult experiment studies reaction behavior of enzymes from the initial reaction to reaching a steady state.
Progress Curve Experiments: Used in enzyme kinetics, this studies enzyme reactions after the initial reaction.
Initial Rate Experiments: The initial rate of specific reactions of enzymes mixed with substrates.

Figure 4: Enzyme Activity Experiments are a Common Use of Proteins (Science Direct)
Western Blot: Also known as a protein immunoblot, Western Blot uses gel electrophoresis to detect a single specific protein in a sample of tissue or cells. Applications of the Western Blot range from detecting proteins after cloning, to tracking illegal substances in athletes, to diagnosing diseases based on the presence of antibodies.
Gel Electrophoresis: This is a method of separating and analyzing protein based on size and charge. Using a gel base, such as polyacrylamide, proteins can be analyzed. The common types of techniques are SDS-PAGE, QPNC-PAGE, and 2-D gel electrophoresis.
Finding Biomarkers: Biomarkers are indications of certain biological processes taking place, such as tracking disease or measuring therapeutic intervention. While still a developing field, the process from discovery to commercialization is an innovative but costly process, as Figure 5 presents.
BioChain’s Protein Extraction Kits
CNMCS Compartmental Protein Extraction Kit: This rigorously tested kit is prepared with the highest standard of pure chemicals. BioChain’s kit contains enough reagents to recover proteins from up to 5 grams of tissues or 125 million cells. The kit is tested for efficiency using SDS-PAGE and immunoblotting methods on different types of marker proteins present in humans. Additionally, all four fractions of proteins extracted are subjected to further electrophoresis and immunoblotting to ensure quality. The kit extracts four types of proteins:
- Cytoplasmic: these proteins are present in the cytosol of a cell cytoplasm. This liquid area is responsible for processes such as glycolysis or cell division.
- Nuclear: this refers to proteins present inside a cell nucleus, including the ones that form chromosomes.
- Membrane: these proteins form the outside layers of cells and tissues. Their functions include transportation/communication between a cell’s internal and external environment, and attaching to the lipid bilayer of other proteins.
- Cytoskeletal: proteins of this compartment consist of a cell’s structure, such as for support, transport, and division.

CNMCS Kit: Extracting from Tissues. Due to the uneven concentration of proteins across areas, it’s important to choose tissues that would have a higher distribution of proteins for maximum extraction. The initial step when extracting from tissue is to chop the tissue and homogenize it before lysis. This mechanical disturbance breaks the tissue surface and structure down. After centrifuging the tissue, the first protein available is cytoplasmic. Next, using chemical buffers at ice-cold temperatures and further centrifugation, nuclear proteins become available. More chemical buffer is added, and the third centrifuge rotation provides membrane proteins. Finally, with the aid of additional buffer, the remaining pellet can be centrifuged a final time for cytoskeleton proteins.
CNMCS: Extracting from Culture Cells. This method is different than extracting from tissues since cells only require lysis of the external membrane to extract proteins, which reduces some steps. First, the culture cells are centrifuged with ice-cold buffer, then passed through a needle base to disrupt the cell membrane and release cell nuclei. A microscope should be used to monitor the level of disruption. Next, spin the cell mix to release cytoplasmic proteins. The cell pellet is suspended back in ice-cold buffer and centrifuged multiple times to release nuclear proteins. Using a different buffer and centrifuging, membrane proteins are available. Finally, using warm buffer and rotating at room temperature releases the cytoskeleton proteins.
Simplifying Challenges in Protein Extraction. The extraction of proteins is a challenging process that often requires management for precision. Often during the cell lysis process, proteins may become denatured from chemical buffers, and this may result in useless proteins that fail to refold or fold into incorrect shapes. The presence of proteinases may also cause digestion of protein; this needs to be accounted for during purification. Cross contamination also tends to be a significant issue. The BioChain CNMCS Kit is carefully designed and quality tested to avoid these issues, with detailed troubleshooting included in the manual.
Separating complex protein mixtures is often a challenging and laborious process. This kit simplifies the process using ready-to-use reagents and a user-friendly protocol that is efficient. This cost-effective method provides high-quality consistent extractions that can be used in many downstream applications, including SDS-PAGE, Western Blotting, IP, gel mobility shits, and protein assays.
BioChain’s proprietary protocol aids in consistency and standardization, and can be used with fresh or frozen cells A sample workflow for the CNMCS kit typically involves several steps that have been made simple, since extracting different types of proteins needs unique levels of processing.
Total Protean Extraction Kit: This kit comes with an extensive protocol designed for maximum protein extraction. Downstream applications of the high quality protein include SDS-PAGE, 2-D gel separation, isoelectric focusing, Western Blotting, gel mobility shift analysis, DNA-protein interaction studies, antibody production, enzymatic activity assays, protein-protein interaction studies, IP, and tissue-specific protein expression analysis.
The recommended minimum sample for extraction can be as little as 0.1 g tissue or 2.5 million cells, but in certain cases using less reagent can be effective with an even smaller sample. The Total Protein Extraction Kit comes with a protease inhibitor cocktail to prevent protein degradation. Proteins extracted from the kit are intact and can be over 200 kd in mass. No freezing and thawing or sonication is required.

Dr. P Kit, Isolation of RNA, DNA, & Proteins: BioChain’s Dr. P Kit is an efficient system that can isolate RNA, DNA, and protein from the same piece of tissue simultaneously up to 150 times. The kit can be used to extract from up to 5 grams of tissue and provides total protein that can be used for Western Blotting.
Author
BioChain Institute Inc.

