Groundbreaking Cancer Detection Study
There have been tremendous advances in cancer treatment since President Richard Nixon declared war on malignancies in 1971. Despite having treatments such as targeted radiation, estrogen blockers, and CAR-T cell immunotherapies, early detection of cancer remains one of the most important factors. For instance, the five-year relative survival rate for non-small cell lung cancer is 61% when cancer has not yet spread outside the lung. The survival rate drops to 35% once the cancer invades nearby structures, and to 6% once it metastasizes to other organ systems.
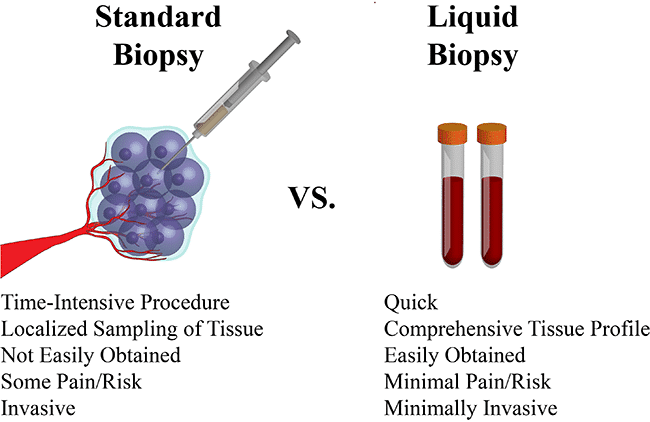
However, early detection of cancer has proven easier said than done, and not without risks. When screening for cancer certain number of patients experience anxiety and undergo unnecessary invasive procedures based on false positives. While some cancers make for relatively easy screening such as cervical cancer via the Pap test, many cancers are inaccessible, and tissue biopsy too invasive for population-level screenings.
The oncologist’s objective is to come up with a less invasive way of screening the healthy population for specific and accurate indications of early-stage cancer. This motivated scientists to develop “liquid biopsy,” where doctors hope to detect tumor-specific protein biomarkers or cancer-associated genetic mutations in circulating free DNA (cfDNA) blood samples.
In an article published in the journal Science, Johns Hopkins researchers Bert Vogelstein and Nickolas Papadopoulos and their colleagues appear to have done exactly that.
A Leap in Early Cancer Detection
A breakthrough was achieved using a liquid biopsy test called “DETECT-A” ̶̶ Detecting cancers Earlier Through Elective mutation-based blood Collection and Testing ̶ which involved more than 9,911 women who had no history of cancer. The study used a multi-cancer blood test, to identify 26 undiagnosed cancers in 10 different organ systems: appendix, breast, colorectal, kidney, lung, lymphomas, ovary, thyroid, uterine and unknown primary site. The tumors were then localized using techniques such as PET-CT, a combination that was 99.6% specific.
Importantly, 17 of the cancers detected by the blood test were diagnosed at an early stage. Twelve of the 26 patients were in remission and eight were still in treatment or had stable disease nine months post-diagnosis.
This was a landmark result for the liquid biopsy field and the industry took note. A more advanced version of the multi-cancer blood test known as CancerSEEK had been licensed by Johns Hopkins to Thrive Earlier Detection Corp for development. On Oct. 27, Exact Sciences announced it would acquire Thrive for $2.15 billion.
Foundational Technology
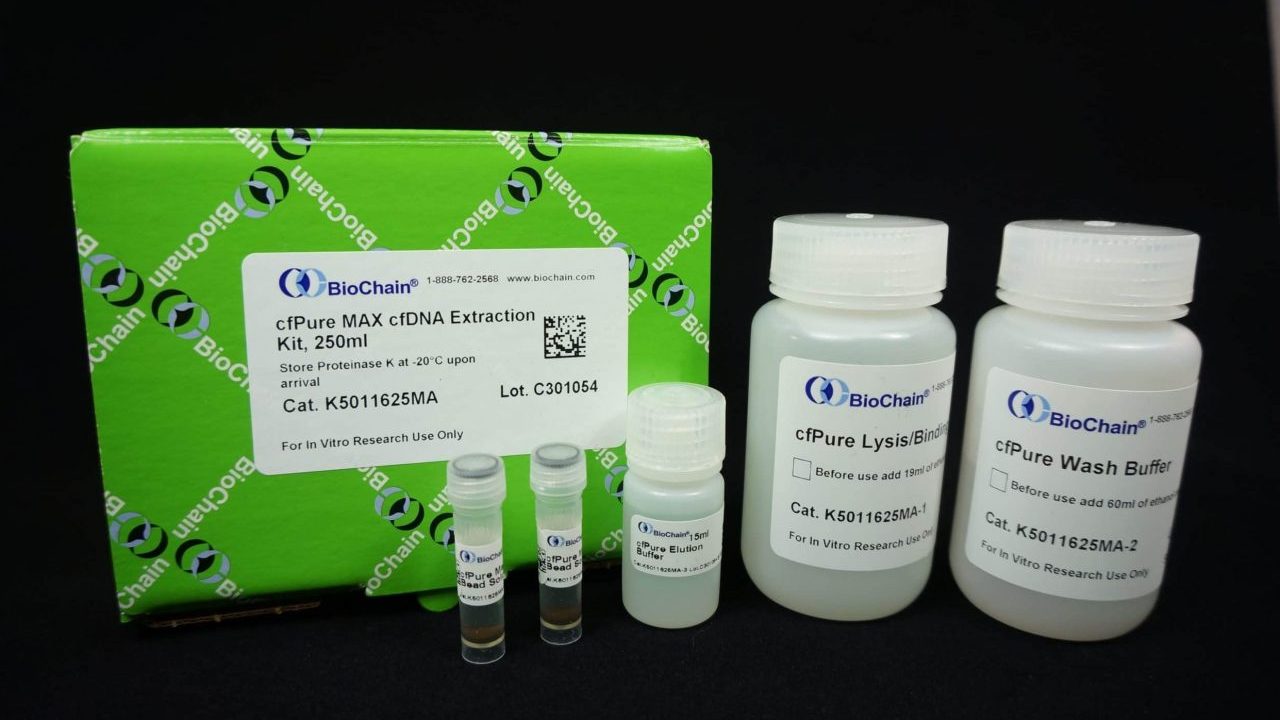
Although much of the liquid biopsy breakthrough has come from next-generation sequencing (NGS) and machine learning, the fundamentals of the field are still focused on an accurate and successful sampling process. If there is no adequate sample in the first place, no algorithm can support it. The DETECT-A study made use of the cfPure extraction kit from BioChain, a magnetic bead-based technology that demonstrated high yields in extracting cfDNA from blood plasma. “In order to get that cfDNA, you need to have the right tools,” says BioChain’s Production and R&D Manager Dr. Franklin Chin, “and we have them. Our extraction kit is the first step in any research that involves cfDNA biomarker discovery”
The cfPure kits are available from BioChain in three different sizes for the extraction of a maximum of 250 ml of cfDNA from human plasma samples. The kit has two different versions: the original cfPure kit which maximizes yield, and the “V2,” which minimizes carryover of genomic DNA into the final elution.

Dr. Bert Vogelstein and his colleagues used the original version of cfPure kits, and the fact that such pioneering researchers used BioChain's technology in a groundbreaking study to process tens of thousands of samples is a source of pride for BioChain. “Dr. Vogelstein is one of the pioneers of liquid biopsy research,” Chin says, “that he is using our kit is pretty big for us.”
Detection of early-stage cancer using the DETECT-A test is just the first of many breakthroughs BioChain hopes their technology will enable. Beyond early diagnosis, for instance, there are efforts at precision tumor treatment and companion diagnostics, according to Chin. Many cancers are drug-resistant, and that resistance is based on their mutational profile. Pairing the cfPure kit with NGS using specific biomarker panels can help identify the mutational landscape for a patient, indicating the therapies best suited to treating their specific cancer.
Chin also hopes to see BioChain’s technology used in the creation of biomarker panels to help identify mutations with a strong correlation to oncogenesis. “You need to have a solid foundation,” he says. “You need to collect as many samples as possible, analyze them, and come up with a panel of biomarkers.” That will expand the reach and accuracy of liquid biopsy, and, ultimately, the success of cancer treatments. That’s something Chin and BioChain are excited to be a part of.
BioChain's Menu of Research Solutions
Whatever your research needs, BioChain offers a cfPure cell-free DNA extraction kit providing market-leading yields for the best possible input for NGS or other processing.
BioChain also offers blood plasma and tissue samples for researchers who need inputs to develop and test their technologies and offers the cfPure cell-free DNA extraction process as a service for those who cannot perform the extractions themselves.
Click here to read more about cfPure kit at BioChain Institute.
Author
Dr. Vidyodhaya Sundaram Ph.D, MBA

