
An Introduction to Detecting Gene Expression
Understanding gene expression in cells is a vital process. Its uses involve detecting diseases such as cancer and Hodgkins, identifying cell death and reproduction, and profiling the distribution of proteins in a cell. There are various methods involved in immunostaining frozen tissue.
One of the most widely used of these methods is immunoflourescent staining, which uses fluorescent dyes to stain certain molecules. However, this type of staining has distinct disadvantages. A significant one is photobleaching, which causes destruction of the molecules being observed. Additionally, it also limited purely to dead cells, unless painstaking and time-consuming procedures are used. Another drawback of immunoflourescence is that certain molecules may be too large to dye inside a cell. As a result, alternative methods must be used.
Two Methods of Detecting Gene Expression in Frozen Tissues
Method 1: ImmuChem IHC Detection Kit
This kit is exceptionally useful for increased sensitivity with expression in tissues. The kit is also convenient, as it includes all components needed for staining. It is important to note that the specific antibodies included in this kit are for mice; antibody purification agents are available too.
Part 1
- First, let the tissue slide thaw and soak in water for 5 minutes.
- Next, transfer the slide to .3% H2O2 for about 10-30 minutes. Be cautious of the incubation time; if they incubate too long, the tissue might slide off the glass.
- Rinse slide with water, then with 1xPBS (with a pH of 7.4).
- Circle the tissue area with a Pap Pen to make it easier to find later.
- Incubate the slides with a mixture of 35 microliters Normal Serum with 3.5 milliliters of 1xPBS for 30 minutes.
- Remove serum from the slides, and incubate the sections with 1xPBS diluted antibody using a humid chamber. This can be done from 1 hour to overnight.
Part 2
- Next, rinse the slides with 1xPBS 3 times for 5 minutes each.
- Mix 1.4 milliliters of 1xPBS with a pH of 7.4 with about 35 microliters of biotinylated anti-mouse IgG. Incubate with this PBS diluted secondary antibody for 30 minutes.
- Again, rinse in 1xPBS 3 times for 5 minutes each.
Part 3
- Now we will prepare the detection solution. This is done by mixing 1.33 milliliters of 1xPBS with 35 microliters of Solution A and 35 microliters of Solution B.
- Incubate for 30 minutes at room temperature, and add it the tissue section previously circled on the slide.
- Incubate the slides for another 30 minutes.
- Once more, rinse the slides at 1xPBS 3 times for 5 minutes each.
Part 4
- To make the development solution, mix 1.6 milliliters of DAB buffer and 35 microliters of Liquid DAB.
- Cover the tissues with this solution and incubate anywhere from 5 to 30 minutes.
- After the time is up, soak the tissue in water.
- The next step would be counter staining, but this is optional. Counter staining would also help remove any residual buffer salts.
- Then, dehydrate the tissue by soaking in a series of alcohols for 3 minutes each: 70%, 80%, 90%, 95%, 100% (I), 100% (II).
- Next, incubate in Xylene for 10 minutes twice.
- Finally, mount the slides.
As a final note, add 2 drops of the detoxification solution in the development solution we created, and incubate for a couple of hours before discarding it.
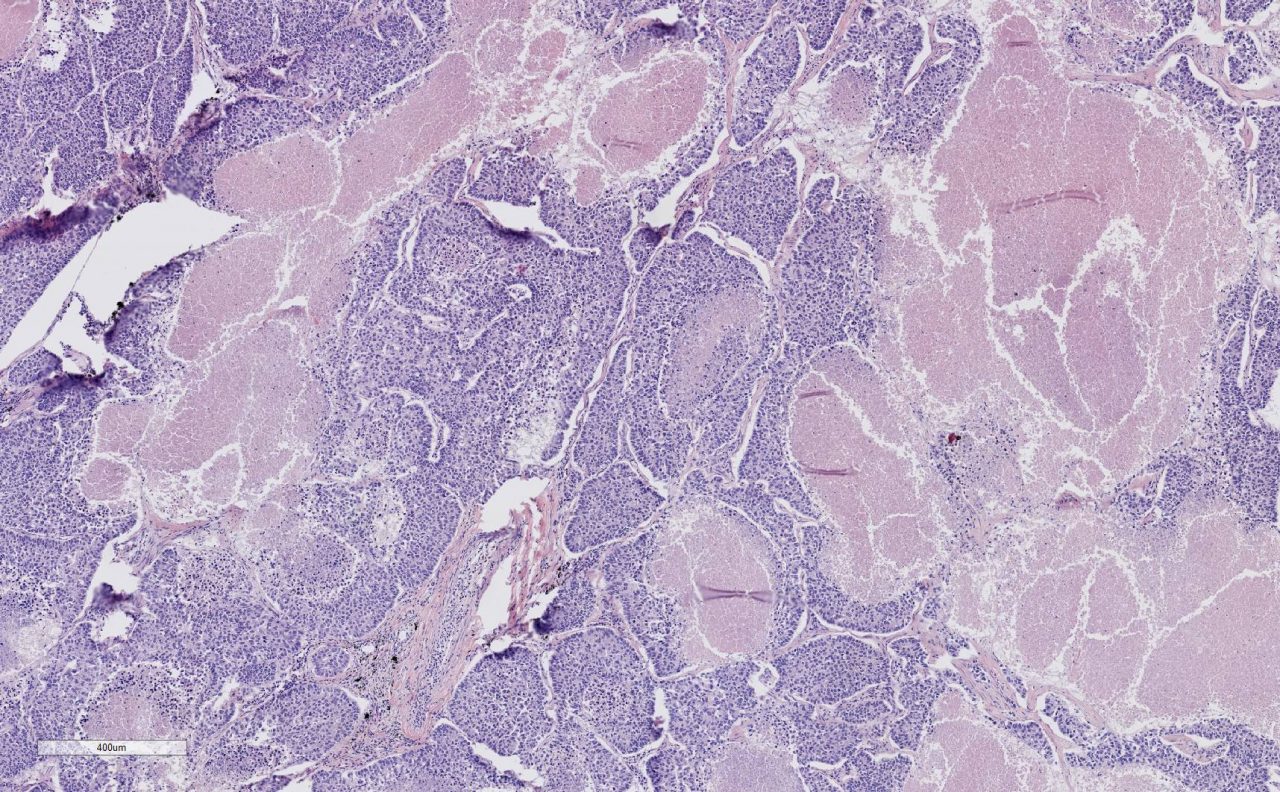
Lung Tumor Tissue 5x Magnification
Method 2: In Situ Hybridization
The advantage of in situ hybridization kit is that it allows the cell to remain as intact as possible, and it is particularly useful if antibodies are not available. The kit includes pre-mixed reagents and is additionally convenient for both frozen and paraffin embedded tissue samples. An additional advantage of the kit is that it is more helpful in finding nucleic acids, such as RNA and DNA. This is why antibodies need not be present for the kit to work.
Part 1
- The first step is to thaw frozen tissue to room temperature and dry it for 15 minutes at 50° C.
- Fix the slides using a 4% paraformaldehyde solution in DEPC-treated PBS for 20 minutes.
- Wash twice with the DEPC-treated PBS for approximately 5 minutes, and then treat the slides with 10 micrograms of Proteinase K for about 15 minutes at 37° C.
- Wash the slides once more with DEPC-treated PBS, and use the previous paraformaldehyde solution to fix the slides for 15 minutes.
- When complete, rinse with DEPC-treated water.
Part 2
- Now the slides must be hybridized. Do this by first using the pre-hybridization solution at 50° C.
- Let incubate for 3-4 hours.
- Then, use the hybridization solution with the digoxingenin labeled probe and this time, let incubate overnight at 45° C.
Discard the solution and rinse the slides with distilled water. If counterstaining is necessary, this would be the time do so. If not, the slides are ready to be mounted.
Part 3
- Next, we will wash the slides with various solutions. The first of these is with 2x SSC, 10 minutes, 45° C.
- Second, wash with 1.5x SSC, 10 minutes, 45° C.
- Third, wash twice with .2x SSC, 20 minutes both times, 37° C.
- At last, incubate the washed slides with diluted blocking solution for 1 hour. Be aware that longer incubation may result in poorer results, so use best discretion.
Part 4
- Finally, the slides are ready to be stained. This is done by incubating the slides with a 1:100 ratio of PBS diluted with antibodies for a few hours (this can be done overnight) at 4° C.
- Wash the slide with 1xPBS 3 times for 10 minutes each.
- Wash twice more with 1x Alkaline Phosphate buffer for 5 minutes both times.
- Create a solution of 6.6 microliters of NBT and 3.3 microliters of BCIP; mix this into 1 milliliter of 1x Alkaline Phosphate buffer, and incubate the slides 2-20 hours.

FFPE Tissue Array

Frozen Tissue Panel

Frozen Tissue Section
BioChain: Ease and Convenience
BioChain's in situ hybridization kit is designed for digoxygenin-labeled probes and alkaline phosphatase detection, eliminating the need for radioactive DNA labeling. The kit includes the pre-hybridization and hybridization solutions, NBT and BCIP detection reagents, blocking solution, SSC buffer, alkaline phosphatase buffer, and an anti-Dig antibody. The in situ hybridization kit does not include reagents and enzymes for labeling probes. Any Dig labeled detection probe can be used. BioChain’s in situ hybridization kit has protocols for both frozen and formalin fixed paraffin embedded (FFPE) tissues and cells.
Features specific to the in-situ hybridization kit:
- Useful for microRNA detection
- Non-isotopic, colorimetric detection of gene expression
- RNase-free, ISH-optimized reagents
- Can be used with either oligonucleotide or ribonucleotide probes
- All lots are tested by ISH using QC-tested probes on known tissues.
- Fully optimized protocol
- Can be used on tissue section and/or tissue microarray slides
Author
BioChain Institute Inc.

