BRCA Gene Mutations and Hereditary Cancer Risk
Breast cancer is the most common type of cancer in women after skin cancer; in the general population, 1 in 8 women (about 13%) will develop breast cancer in their lifetime. While many factors may increase the risk of cancer, such as smoking or exposure to chemicals or radiation, about 5-10% of cancer cases are associated with inherited genetic mutations (National Cancer Institute, 2019). Genes called BRCA1 and BRCA2 (short for BReast CAncer gene) were the first genes to be associated with a strong family history of breast and ovarian cancer.
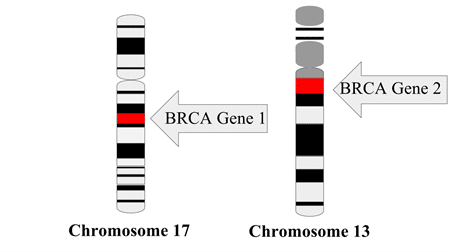
Everyone has the BRCA1 and BRCA2 genes (Figure 1 Wikimedia Commons), which code for proteins that normally serve important functions in repairing damaged DNA. But women with certain mutations in the BRCA1 gene have up to a 72% lifetime risk of breast cancer and 44% risk of ovarian cancer (compared to a 1% risk of ovarian cancer for the general population), while women with BRCA2 mutations have up to a 69% lifetime risk of breast cancer and 17% risk of ovarian cancer (National Cancer Institute, 2020). BRCA2 mutations also increase the risk of breast and prostate cancer in men. Individuals with BRCA gene mutations tend to be diagnosed with more aggressive cancer at a younger age, often in their 30s and 40s.
Genetic Testing and Personalized Medicine
Because people with BRCA gene mutations have such significantly increased risks of breast and ovarian cancer, testing for BRCA gene mutations can be an important step in personalizing cancer screening and risk management based on genetic risk factors. Individuals with a strong family history of breast and/or ovarian cancer may be referred by their doctor to a genetic counselor, who will take a detailed family medical history and use mathematical models to estimate that individual’s personal cancer risk. Based on the results of this cancer risk assessment, the genetic counselor may recommend testing for mutations in the BRCA genes and possibly other additional genes associated with increased risk of breast and ovarian cancer.
Women who test positive for BRCA gene mutations typically undergo more frequent screening for breast cancer, usually involving both a mammogram and an MRI every year. Some women with BRCA gene mutations and a strong family history of cancer decide to undergo preventive mastectomies and oophorectomies (removal of ovaries) to reduce their future risk of developing cancer. Angelina Jolie notably raised public awareness about risk-reducing surgery when she discussed her personal decision to have a preventive mastectomy given her BRCA1 mutation status (Jolie, 2013).

The Promise of Next Generation Sequencing
To test for BRCA gene mutations, a patient sample, typically a blood sample, is sent to a lab that specializes in genetic testing. The goal of genetic testing is to examine the DNA sequence of the gene to determine if any harmful mutations are present. Traditionally, this has involved amplifying many copies of the gene of interest ( BRCA1 or BRCA2 in this case) using the polymerase chain reaction (PCR), followed by Sanger sequencing using fluorescently-labeled nucleotides to determine the DNA sequence. While Sanger sequencing is still considered the gold standard to confirm DNA mutations, it can be time-consuming to sequence large genes. The length of a typical Sanger sequencing read is approximately 1 kb, while the BRCA1 gene is 5.6 kb in length and BRCA2 is 10.3 kb (Nicolussi et al., 2019).
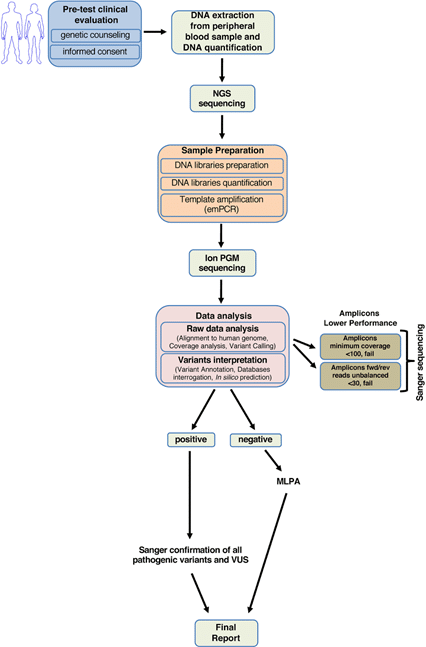
Recent advances in DNA sequencing technology have allowed scientists to utilize next generation sequencing (NGS) as an alternative approach to traditional Sanger sequencing methods for detecting genetic mutations (Nicolussi et al., 2019, Figure 2). While Sanger sequencing reads a single DNA sequence at a time, an NGS approach first breaks the DNA into many smaller fragments (200-500 bp), to which specific identifier tags are added at the ends to create a library of DNA fragments from the gene of interest. Each DNA library uses unique tags that tie the sequences back to specific genes from a specific patient. Millions of DNA fragments from multiple sample libraries (e.g. multiple patients being tested for both BRCA1 and BRCA2 mutations) can then be amplified and sequenced at a time using a high-throughput NGS platform.
The DNA sequences of overlapping fragments are then aligned using software to assemble the DNA sequence of the entire gene. Because NGS can sequence many DNA samples simultaneously, using NGS technology for genetic testing can increase the number of different genes and patient samples that can be tested while decreasing turnaround time and sequencing cost per sample, enabling doctors and patients to receive BRCA genetic testing results more quickly and cost effectively.
BioChain Launching New BRCA-Characterized Breast Cancer Tissue Lines
Stay tuned for BioChain’s upcoming blog on its new line of BRCA1- and BRCA2- characterized breast cancer tissue samples! These tissue samples have been characterized for BRCA1 and BRCA2 genetic mutations using NGS, and can be used in genetic testing validation, diagnostic assay development, cellular localization studies, and many other applications.
BioChain offers many other breast cancer-related products, including tumor tissue arrays with normal tissue controls, triple negative breast cancer tissue samples, and CancerSeq tumor tissue lines prescreened by NGS for single nucleotide polymorphisms, insertions, deletions and copy number variation. BioChain also offers custom procurement and characterization services to meet specific research needs.
References
- The Genetics of Cancer. (2019, March 15). National Cancer Institute.
- BRCA Gene Mutations: Cancer Risk and Genetic Testing. (2020, November 19). National Cancer Institute.
- Jolie, A. (2013, May 14). My Medical Choice. The New York Times, A25.
- Nicolussi, A., Belardinilli, F., Mahdavian, Y., Colicchia, V., D'Inzeo, S., Petroni, M., Zani, M., Ferraro, S., Valentini, V., Ottini, L., Giannini, G., Capalbo, C., & Coppa, A. (2019). Next-generation sequencing of BRCA1 and BRCA2 genes for rapid detection of germline mutations in hereditary breast/ovarian cancer. PeerJ, 7, e6661..
- Park, Alice. “The Angelina Effect: Her Preventive Mastectomy Raises Important Issues.” Time, Time, 27 May 2013
Author
Dr. Lianna Wong received her Ph.D. in Molecular and Cell Biology from the University of California, Berkeley. She has 20 years of biology education experience at Santa Clara University and UC Berkeley.


